An enzyme recently unearthed from the depths of the ocean is capable of breaking down PET plastics, an important discovery in the fight against the plastic pollution that plagues our coasts and oceans.
The groundbreaking discovery came from a collaborative study led by the research team of Prof. Ruth Schmitz-Streit at the University of Kiel, Germany, with significant contributions from researchers at the University of Hamburg and the Heinrich Heine University of Düsseldorf.
This study represents a key advance in our understanding of the PET-degrading enzyme, its potential mechanisms, and its evolutionary significance in global marine ecosystems. These groundbreaking results have been published in the journal Communication Chemistry, where the team not only explored potential biotechnological applications, but also emphasized the far-reaching significance of this enzyme in biogeochemical processes in both marine and terrestrial environments.
What sets this PET-degrading enzyme apart is its unique source.
Prof. Ruth Schmitz-Streit, head of the working group on microbial molecular biology at the Institute for General Microbiology (IfAM) and a member of the Kiel Marine Science (KMS) research priority area at Kiel University, explains, "In our study, we revealed a new genetic resource from deep-sea archaea, which was previously in this case unknown."
To date, most PET-degrading enzymes, of which there are about 80, are primarily associated with bacteria or fungi.
"Our findings contribute significantly to a better understanding of the ecological roles played by deep-sea archaea and their potential impact on PET waste degradation in the marine environment," the microbiologists added.
This newly discovered enzyme, named PET46, was identified and fully characterized using a macro-genomic approach. The team isolated the gene from deep-sea samples based on genetic similarity to known sequences, synthesized the corresponding coding gene, produced the enzyme in E. coli bacteria, and meticulously studied its biochemical and structural properties.
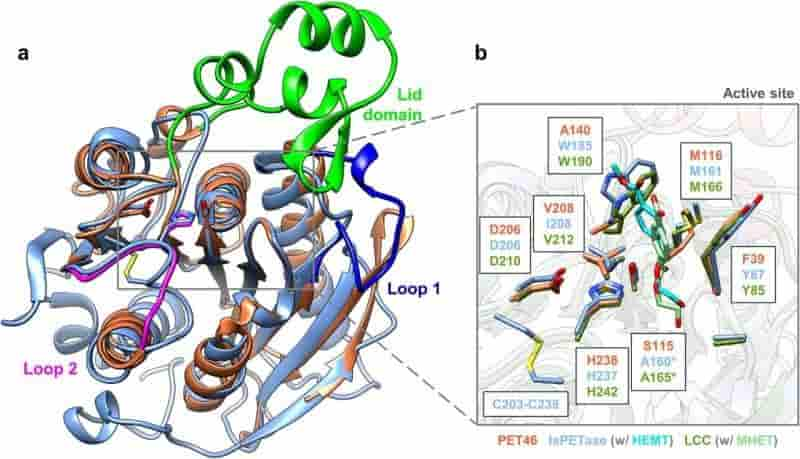
PET46 exhibits a variety of unique properties that extend the range of PET-active enzymes. Its structural differences from previously documented enzymes are particularly noteworthy.
For example, PET46 exhibits a remarkable ability to break down long-chain PET polymers and short-chain PET oligomers that are capable of sustained degradation.
One of the outstanding features of this enzyme is its novel substrate binding mechanism, distinguished by a unique "lid" of 45 amino acids located above the enzyme's active center. In contrast, other PET enzymes typically use aromatic amino acids for binding near their active sites.
At the molecular level, PET46 shares remarkable similarities with another enzyme called ferulic esterase, which naturally degrades lignin in plant cell walls. Given the structural similarity between lignin and PET, these naturally occurring PET-degrading enzymes may play an important role in wood decomposition, such as in forest soil composting.
PET46's extraordinary biochemical properties make it a promising candidate for a variety of applications, including marine and terrestrial plastics and biotechnology. Notably, PET46 exhibits excellent efficiency at 70°C compared to reference enzymes from bacteria and composting plants that function optimally at their respective temperatures.
The groundbreaking research was conducted under the auspices of the PLASTISEA project led by Prof. Ute Hentschel Humeida of the GEOMAR Helmholtz Centre for Marine Research in Kiel. Dr. Jennifer Chow of the University of Hamburg and Dr. Pablo Pérez-Garcia, a research assistant in the Schmitz-Streit group, contributed jointly as first authors of the study.

