One of the major environmental issues facing the planet today is the rising level of plastic consumption and waste. According to a recent OECD study, 460 million tons of plastic were produced globally in 2019, and consumption will continue to grow despite the expected increase in the deployment of recycling technologies.
With carbon dioxide (CO2) emissions also soaring, the emerging carbon capture and utilization (CCU) industry is proposing a solution to both problems: using CO2 emissions as a feedstock to make low-carbon, biodegradable polymers.
The recent IDTechEx (Cambridge, UK) report "Carbon Dioxide (CO2) Utilization 2022-2042: Technologies, Market Forecasts and Players" analyzes the opportunities and challenges of creating this proposed circular carbon economy.
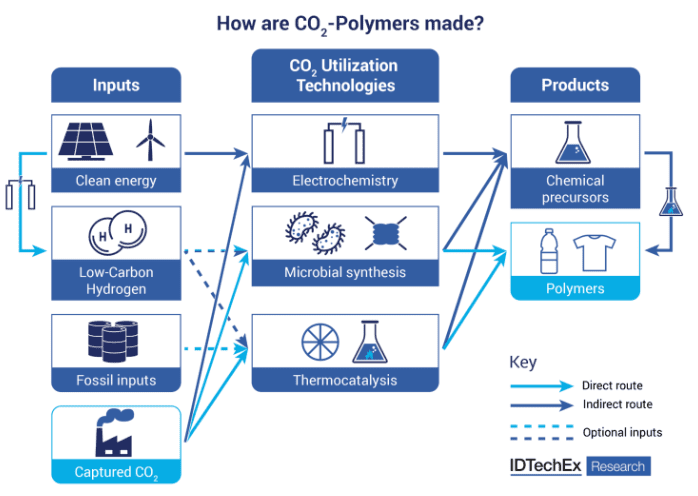
How do you make polymers from CO2?
At least three main pathways exist for the conversion of CO2 into polymers: electrochemical, bioconversion and thermocatalytic. The latter is the most established CO2-utilization technology, where CO2 can be used directly to produce CO2-based polymers, most notably biodegradable straight-chain polycarbonates (LPC), or indirectly by producing derivatives of chemical precursors (e.g., structural units such as methanol, ethanol, acrylates) or monoethylene glycol [MEG]) for polymerization reactions.
LPCs made from CO2 include polypropylene carbonate (PPC), polycarbonate ethylene (PEC) and polyurethane (PUR). PUR is a major market for CO2-based polymers for applications in electronics, mulch, foams and the biomedical and healthcare sectors.
CO2 can contain up to 50% by weight of a polyol, which is one of the main components in PUR.CO2-derived polyols (alcohols with two or more reactive hydroxyl groups per molecule) are made by combining CO2 with an epoxide, an oxygen-containing ring-like molecule called an epoxide. The polyol is then combined with an isocyanate component to prepare PUR.
Companies such as Econic (Amsterdam, The Netherlands), Costron (Leverkusen, Germany) and Aramco Performance Materials (Dhahran, Saudi Arabia) (IP from Novomer - Rochester, NY, USA) have developed novel catalysts to facilitate the manufacture of CO2 based polyols. Fossil inputs are still required through this thermochemical pathway, but manufacturers can replace some of them with waste CO2, potentially saving on raw material costs.
In the field of emerging technologies, chemical precursors for CO2 based polymers can be obtained by electrochemical or microbial synthesis.
While electrochemical conversion of CO2 into chemicals is at an early stage of development, biological pathways are more mature and have reached early commercialization. Recent advances in genetic engineering and process optimization have led to the use of chemoautotrophic microorganisms in synthetic biopathways to convert CO2 into chemicals, fuels and even proteins.
Unlike thermochemical synthesis, these biological pathways typically use conditions close to ambient temperature and pressure, which may be less energy intensive and costly at scale. Notably, California-based startup Newlight (Huntington Beach, USA) is bringing to market a direct biological pathway that converts its microbial conversion of captured CO2, air and methane into polyhydroxybutyrate (PHB), an enzymatically degradable polymer.
The current scale of CO2-based polymer manufacturing is still small compared to the existing petrochemical industry, but there are already successful commercial examples. One of the most available products is aromatic polycarbonate (PC) made from CO2, developed by Asahi Kasei (Tokyo, Japan) in Taiwan since 2012. Recently, the US company LanzaTech (Skokie, IL) successfully worked with Unilever (London, UK), L'Oréal (Clichy, France), On (Zurich, Switzerland), Danone (Paris, France), Zara (Arteixo, Spain) and Lulumelon (Vancouver, Canada) to convert carbon emissions captured in industrial processes into polymer precursors - ethanol and MEG - for the manufacture of packaging items, shoes and textiles using microorganisms.
The problem still exists
While the idea of reusing waste greenhouse gases as raw materials may seem like a win-win proposition, there are many viable utilization pathways that arise for each type of CO2. Will it really lead to emissions reductions? Not all CO2-use pathways are equally beneficial to climate goals, and not all pathways are economically scalable. Scarce resources with alternative uses must be allocated where they have the greatest potential to generate economic value and mitigate climate change. As the world's appetite for plastics does not seem to be abating, a circular carbon economy can help sustain lifestyles by fostering a petrochemical industry that sees waste CO2 as a viable feedstock.

