Although recycling and reuse have always been strongly advocated, in reality there are many simple and low-power small electronic devices that are often disposable. Because of this, "disposable batteries" are also one of the areas of interest for scientists.
Recently, scientists at the Swiss Federal Institute for Materials Science and Technology (Empa) have developed a more environmentally friendly "paper-based battery" that requires only water to activate and is biodegradable once discarded.
The researchers said the battery could be used in applications such as "smart" shipping tags, environmental sensors and disposable medical diagnostic devices.
It is reported that in the current proof of concept, the battery consists of multiple connected cells. Each cell is 1 cm2 in size and its paper substrate is impregnated with sodium chloride (also known as table salt). It has a waxy coating on one end and two wires attached to it.
Specifically, an ink containing graphite flakes is printed on one side of the paper, which serves as the cathode. The other side is printed with an ink containing zinc powder, which serves as the anode. On both sides of the paper, covered with these two inks is a third ink containing graphite flakes and carbon black - which connects the cathode and anode to the two wires mentioned above.
Once a small amount of water is applied to the cell, the liquid causes the sodium chloride in the paper to dissolve, releasing charged ions. As these ions diffuse in the wet paper electrolyte, they cause the zinc in the anode to be oxidized, releasing electrons.
In a laboratory test, two such batteries successfully powered a small alarm clock with an LCD display.
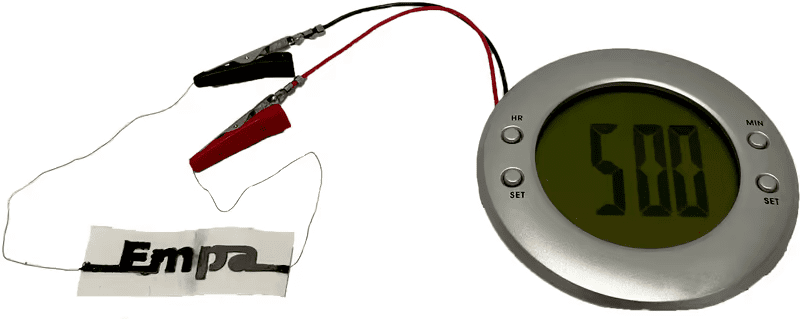
Empa explains, "These electrons can be transferred from the zinc-containing anode, through ink, wires and devices containing graphite and carbon black, to the graphite cathode, where they undergo a redox reaction with the oxygen in the environment to produce an electric current and power external devices."
Remarkably, the researchers found that just two drops of water was enough to activate a battery in 20 seconds. When not connected to an energy-consuming device, this battery reaches 1.2 volts. The battery's performance drops significantly after an hour as the paper dries out. But with two more drops of water, it can work for another hour at 0.5 volts.
This research has been recently published in the journal Scientific Reports. Empa Chief Scientist Professor Gustav Nyström believes that with further engineering, the drying of paper should not be a limiting factor for such a battery. He has previously developed a biodegradable microcapacitor.

