Polyethylene terephthalate (PET) is a very common polymer used in everyday life, often as an outer packaging material for mineral water bottles, beverage bottles, fruit and salad packaging and various household appliances.
The amount of PET waste currently accounts for 12% of the total global waste. And most of PET products are disposable consumer goods. The piles of waste PET materials are not easily degradable in a short time and will cause environmental pollution. Researchers discovered enzymes capable of degrading PET back in 2005, and combining them with downstream green processes can convert PET monomers into value-added chemicals, promising sustainable recycling of PET.
PET hydrolases are generally isolated from microbial colonies, but highly active PET hydrolases that can withstand high temperature and high salt environments and are pH tolerant are still rare.
PET hydrolases are generally isolated from microbial colonies, but highly active PET hydrolases that can withstand high temperature and high salt environments and are pH tolerant are still rare.

A new polyethylene terephthalate (PET) hydrolase, MG8, was discovered in human saliva by researchers including Chayasith Uttamapinant at the VISTEC Institute of Science and Technology in Thailand.
MG8 exhibits potent PET plastic degradation activity under different temperature and salinity conditions, outperforming some naturally occurring engineered hydrolytic enzymes. The research team has also converted PET hydrolases into covalent binders that can be used for biofunctionalization of PET, which is expected to greatly expand the application areas of plastic products.
The research was published in Angewandte Chemie International Edition under the title of discovery of polyethylene terephthalate (PET) hydrolase from human saliva metabolome and its genetic code extension for PET degradation and biofunctionalization.
[Source of PET hydrolase].
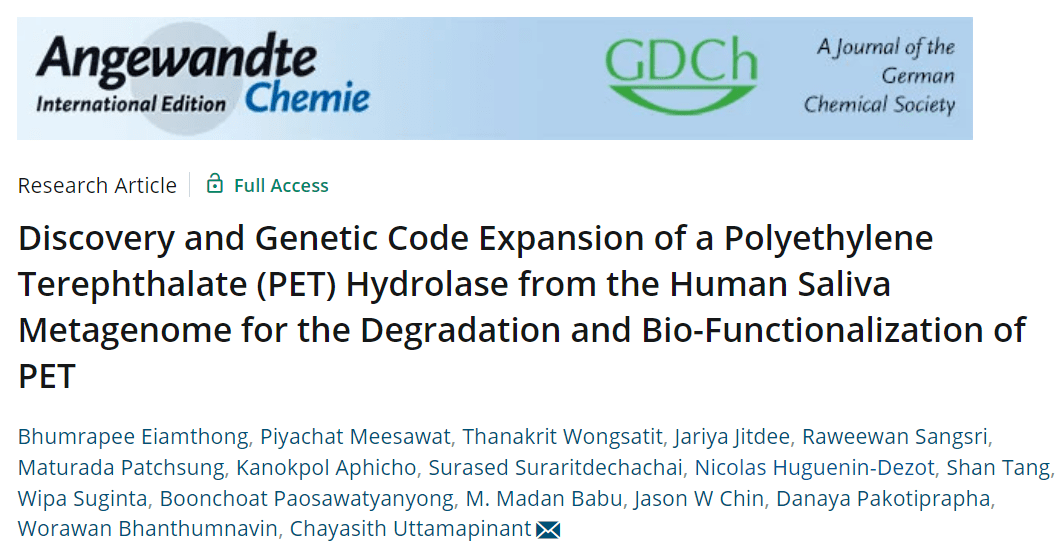
PET has been used as a food packaging plastic for decades, which has the potential to induce the production of hydrolytic enzymes in the human digestive system that can degrade PET. To test this hypothesis, the researchers searched MGnify, a public database that collects microbiome data from different environmental sources, which contains >140,000 human samples and >45,000 samples from aquatic systems, about 75% of which are from marine systems. The high salt environment of human body fluids also favors the production of highly salt-tolerant PET hydrolases while maintaining activity at ambient temperatures similar to their native environment. Finally, seven PET hydrolases from the marine environment and three from the human microbiome were screened for further characterization.
Although all of these hydrolases have similar folding structures, the surface charge (both global and local) is different for each enzyme. Differences in surface charge can greatly affect their substrate recognition ability and catalysis. The MG1-MG7 and MG9 enzymes contain an overall acidic surface charge, with MG1 and MG3 containing acidic active sites, while the remaining marine source candidate enzymes have more neutral active sites.
MG8 is an enzyme from the human salivary macrogenome and is expected to contain many basic residues on its surface, with an overall alkaline isoelectric point, but its active site is neutral. MG10 is from the human skin microbiome and contains evenly distributed acidic and basic residues, so it is close to neutral overall, while containing a neutral active site.
[Characterization of PET hydrolase].
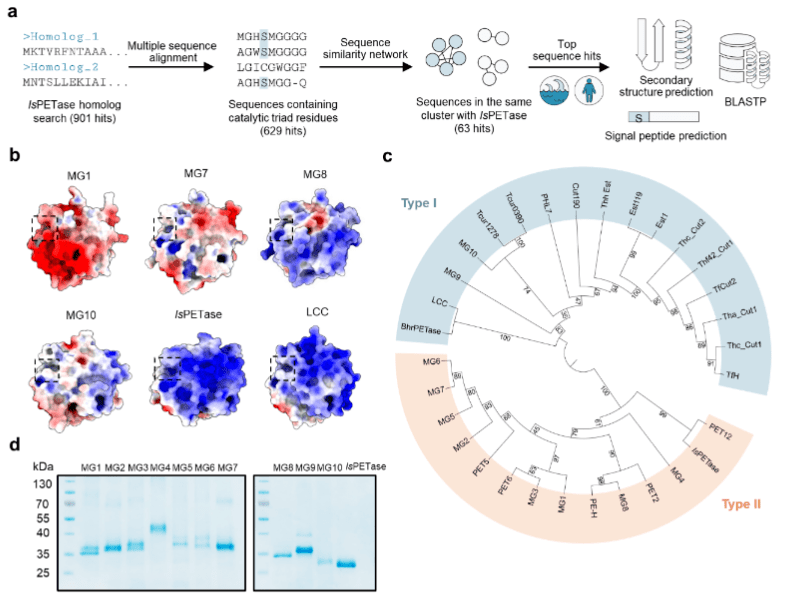
The researchers selected E. coli BL21 (DE3) as the expression host and further cloned, expressed, purified and evaluated the MG1-MG10 enzyme activity. MG1, MG7 and MG8 were the most active in hydrolyzing BHET and showed increased activity at higher NaCl concentrations. However, their BHET hydrolysis activity at 37 °C was at most only 27% of IsPETase activity. This suggests that while BHET hydrolysis may be a prerequisite for the degradation of PET plastics, the hydrolysis efficiency of small molecule BHETs by PET hydrolases alone is not a good predictor of their ability to hydrolyze PET plastics. The researchers then set out to test the efficiency of PETase in hydrolyzing PET powders. At 37°C, MG8 was three times more efficient than IsPETase in degrading PET powder. Further optimization of the reaction temperature further increased TPA yield by 5-7 times and MHET yield by 2 times when MG8 hydrolyzed PET powder at 45-65°C. 55°C is the optimal hydrolysis temperature for MG8, and the enzyme produces 83 times more TPA than IsPETase under its optimal conditions. MG8 outperforms IsPETase in the degradation of PET. Then, the activity of MG8 was further compared with other engineered PETase variants, and MG8 still showed significant advantages with its lower use temperature and higher hydrolytic activity. Furthermore, 55°C is below the melting point of the crystalline region of PET. It is worth mentioning that all PET hydrolases found so far had difficulties in depolymerizing highly crystalline PET, while the crystallinity of PET powder degraded with MG8 remained essentially unchanged (~30%).
[Mechanism of degradation of PET by MG8].
MG8 has a melting temperature (Tm) of 54°C and IsPETase has a melting temperature (Tm) of 42°C. This indicates that MG8 has higher heat resistance. Although the overall pI of MG8 is predicted to be basic (9.23), the acidic amino acid clusters still present on the protein surface as well as hydrated salt ions may help protein hydration to maintain its folded structure and high activity at higher salt concentrations. MG8 is also characterized by its extended sequence RYD, which is not present in all other MGases as well as in other known PET hydrolases. Deletion of the RYD residue from MG8 reduced its PET powder degradation activity by 5-30 fold. In contrast, the addition of RYD residues to IsPETase did not improve IsPETase activity, suggesting that this extended sequence can only exhibit an advantage in MG8. In addition, MG8 purified under non-denaturing conditions showed better hydrolytic activity towards PET powder than refolded MG8, but the latter could be produced in higher yields. MG8 is easy to denature and refold, while being able to maintain good activity and is expected to be produced on a large scale.
[Functionalized application of PET hydrolase].
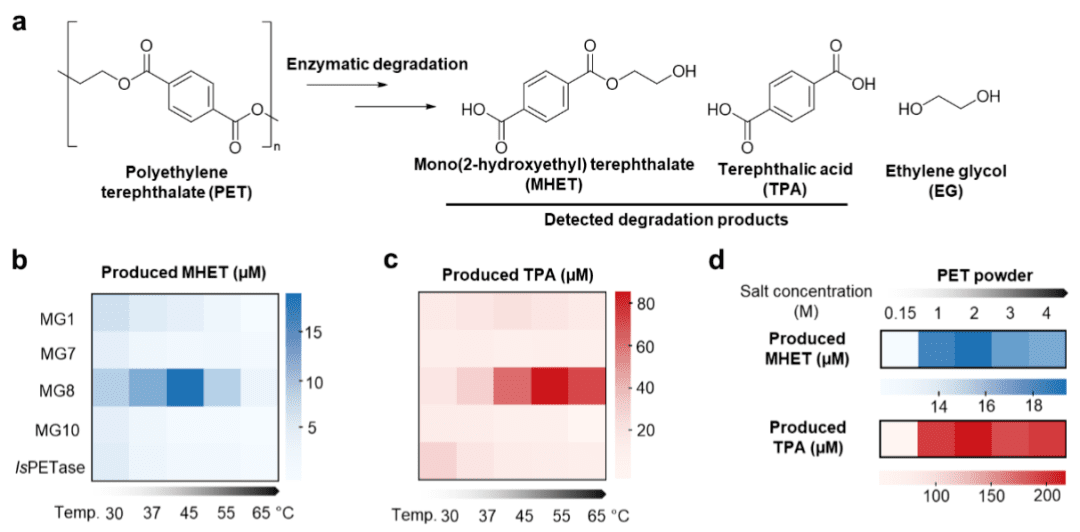
PET is flexible and deformable, making it an important candidate for wearable sensor substrate materials. In addition to clarifying the degradation ability of PET hydrolases, the researchers also verified the feasibility of using MG8 as a tool for PET surface functionalization. During MG8-mediated PET hydrolysis, a transient acylase intermediate is formed between MG8 and PET. Site-specific substitution of MG8 using 2,3-diaminopropionic acid (DAP) allows stable capture of this intermediate. MG8 incorporated with DAP traps PET through amide bonds, thus making the resulting enzyme-plastic complexes highly stable. Therefore, MG8 doped with DAP is expected to be firmly bound to PET as a covalent binder, and then the protein can be immobilized on PET by MG8(DAP) in a way that does not require harsh chemical treatment of the inert plastic surface for subsequent processing, which helps to expand the practical applications of PET-based biosensors.
The researchers also verified the promise of MG8 (DAP) for other aliphatic polyesters such as polybutylene succinate (PBS), polycaprolactone (PCL), and polylactic acid (PLA).
[Conclusion].
Researchers systematically explored macrogenomic data and identified a novel PET hydrolase, MG8, from the human saliva macrogenome. It is the first PET hydrolase identified from the human macrogenome, exhibits potent activity under different temperature and salinity conditions, and outperforms several naturally occurring and engineered PET hydrolases in terms of PET degradation efficiency. And by applying the genetic code extension technology to MG8, the researchers have successfully transformed PET hydrolase into a PET binder that can covalently attach protein carriers directly to PET plastics, completing the functionalization of surface inert PET materials without demanding conditions and complex surface treatment processes. This bioengineered adhesive has become an important tool for extending the functionalization of plastic products, greatly expanding the sensing applications of PET-based flexible sensors in areas such as disease detection.

