Traditional polyhydroxy fatty acid esters (PHAs) are a large class of natural polymeric materials synthesized by microorganisms using renewable glucose and fatty acids as substrates, and over 150 different monomers have been identified.
PHA has biodegradability, biocompatibility, gas barrier properties, thermoplasticity and other characteristics, and has broad application prospects in many fields such as plastic packaging, chemicals, pharmaceuticals, agriculture, bioenergy, etc.
PHA can be rapidly and naturally degraded in both seawater and soil, and its degradation products are naturally occurring energy molecules in the human body and animals, and PHA can also be used as food for animals, making it ideal for solving the plastic pollution problem.
The current synthesis process of PHA is mainly through bacterial fermentation, which requires finding specific strains of bacteria and facing defects such as slow reaction process, low conversion rate of raw materials, easy contamination by bacteria and large investment in equipment. Our domestic PHA producers such as microstructure workshop and blue crystal microorganism have chosen the biological method.
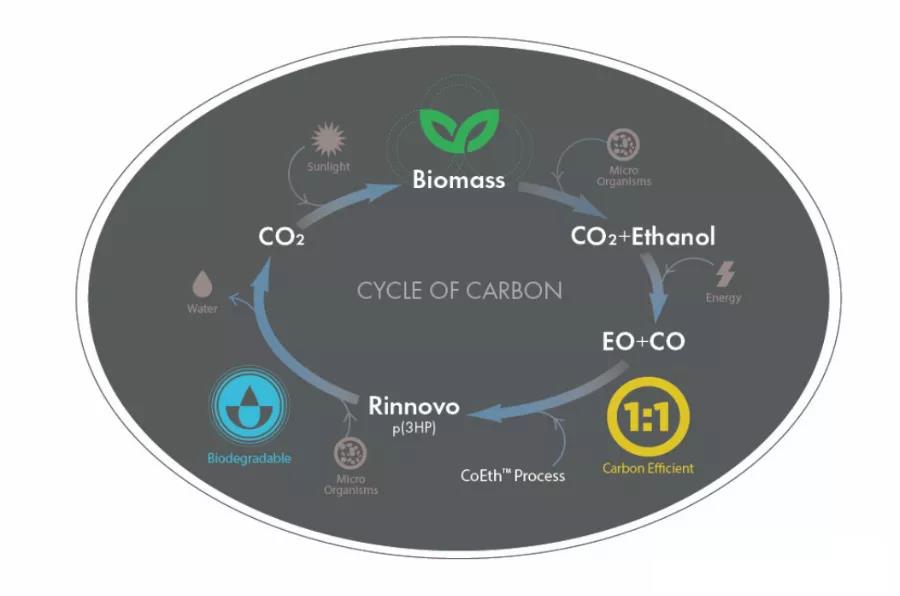
In contrast to the traditional biological fermentation method, the American company Novomer has opted for a chemical synthesis method for the synthesis of PHA.
We have completed the design of a pilot process package with an output of 8 tons/year and are promoting the industrialization technology of producing cyclic lactone monomer/polyhydroxy fatty acid esters by co-polymerization of carbon monoxide and epoxy alkanes, producing carbon dioxide and ethanol through biomass fermentation, and then synthesizing PHA through intermediates by electrochemical action.

It is reported that the direct alternating copolymerization method of epoxy alkane and carbon monoxide has the advantages of cheap raw materials and simple process, and has the prospect of large-scale industrial production based on modern chemical technology.
At present, the methods of producing polyhydroxy fatty acid esters from epoxy alkanes and carbon monoxide can be divided into two categories according to the polymerization reaction mechanism.
One type is the direct alternating copolymerization of epoxy alkanes and carbon monoxide to prepare polyhydroxy fatty acid esters, but the existing catalytic systems, however, face the problem of low polymer selectivity.
The other type is tandem polymerization, i.e., firstly, epoxy alkanes are coupled with carbon monoxide to prepare β-lactones, and then other catalysts are added to achieve ring-opening polymerization of β-lactones to prepare polyhydroxy fatty acid esters.
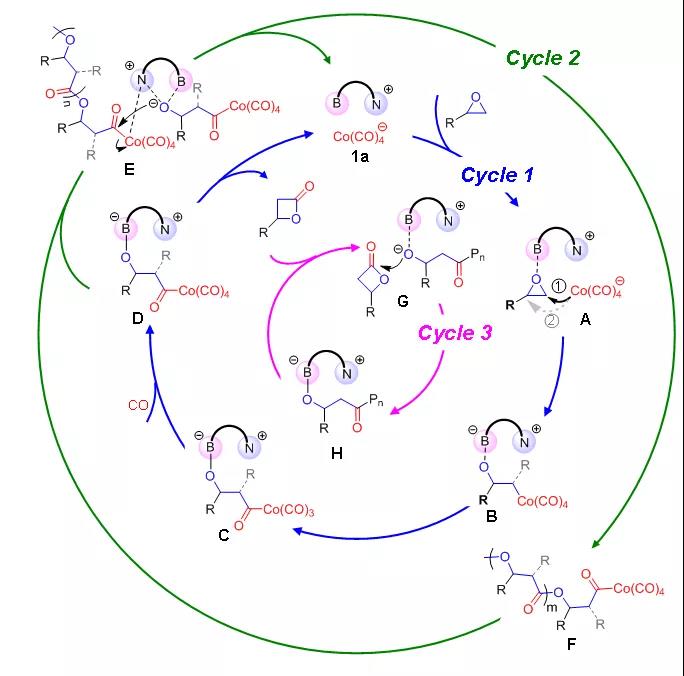
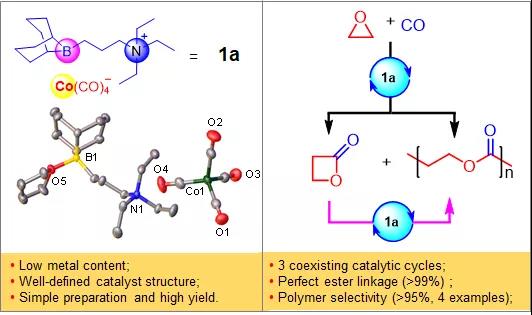
Recently, Wu Guangpeng's group at Zhejiang University in China discovered for the first time a third co-polymerization reaction mechanism: the simultaneous existence of three catalytic cycles (including the ring-expansion reaction of epoxy alkanes to prepare β-lactones, the alternate insertion of epoxy compounds and carbon monoxide to prepare polyesters, and the ring-opening homopolymerization of in situ generated β-lactones) under the action of the same catalyst to achieve efficient synthesis of polyhydroxy fatty acid esters.
Such catalysts and their unique catalytic history enrich the current systems and methods for the preparation of polyhydroxy fatty acid esters from carbon monoxide and epoxy alkanes. They designed organoboron cobalt catalysts with the advantages of simple preparation, high yield, low metal content (only one metal center), >95% polyester selectivity in the resulting products, and applicability to many different epoxy monomers.