PLA is the most widely studied bio-based degradable polymer material. PLA can degrade within weeks under industrial composting conditions, but it is still more difficult to degrade in natural environments such as soil or seawater. The introduction of more degradable bonds by copolymerizing propyl cross ester with other monomers is an effective strategy to improve the degradability of PLA.
Recently, Professor Antoine Buchard et al. at the University of Bath, UK, have copolymerized a novel cyclic xanthanate monomer derived from D-glucose with propylene glycol by ring-opening polymerization (ROP) to obtain an unprecedented UV-degradable PLA copolymer. The work was published in the journal Chemical Communications in a paper entitled "UV-degradable poly(lactic acid) materials by polymerization with sugar-derived cyclic xanthanic acid".
1. Synthesis of cyclic xanthanate monomers
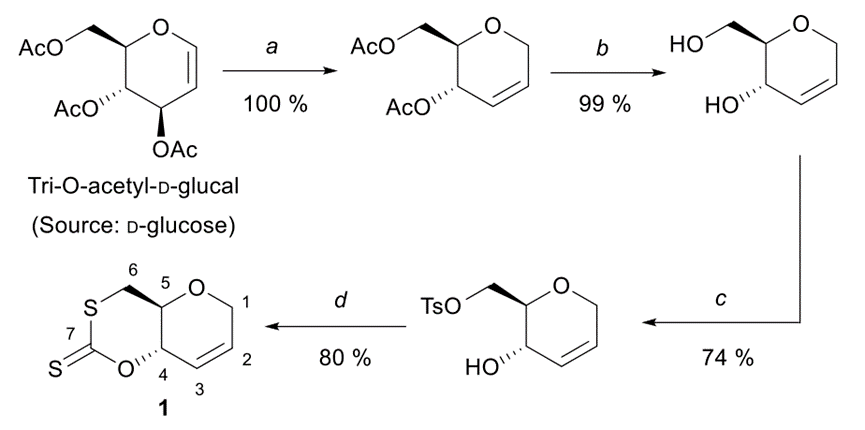
The synthetic route of the cyclic reduced acid monomer from Tri-O-acetyl-D-glucal derived from D-glucose is shown below.
2. Synthesis of xanthanate polymers
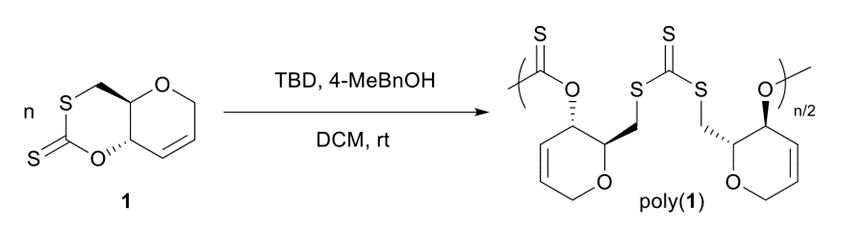
The ROP reaction of cyclic xanthate monomer was carried out at room temperature using 1,5,7-triazabicyclo[4. 4.0]dodec-5-ene (TBD) as catalyst, 4-methylbenzyl alcohol (4-MeBnOH) as initiator, and dichloromethane (DCM) as solvent, and the reaction process and polymer structure are shown below.
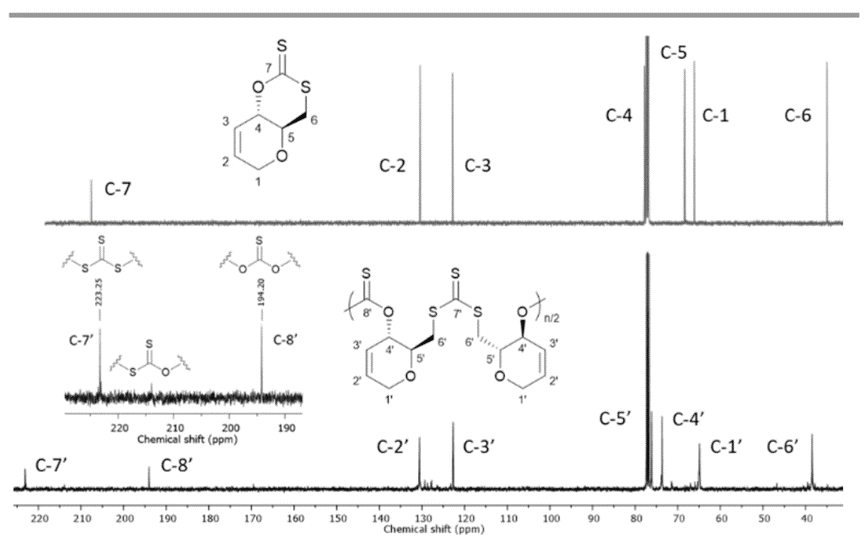
The NMR spectra of cyclic xanthanate monomer and polymer are shown below.
3. Synthesis of cyclic xanthan monomer copolymers with poly(lactic acid)
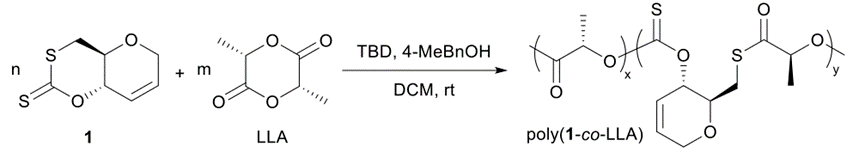
The copolyesters were synthesized using a one-pot copolymerization of cyclic xanthanate monomer and L-propyl cross-ester, as shown in Figure 4, and the NMR hydrogen spectra of the copolymers are shown below.
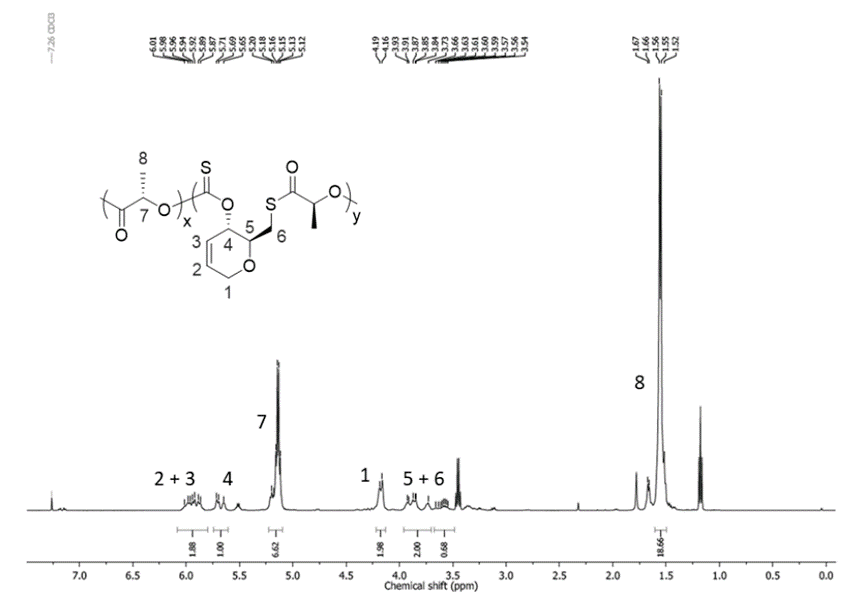
Even with the introduction of only 1 % of cyclic xanthan monomer in L-propyl cross ester, the copolyesters are fully amorphous polymers with Tg in the range of 47-51 °C and initial thermal decomposition temperature Td5% in the range of 187-228 °C.
4. UV degradation
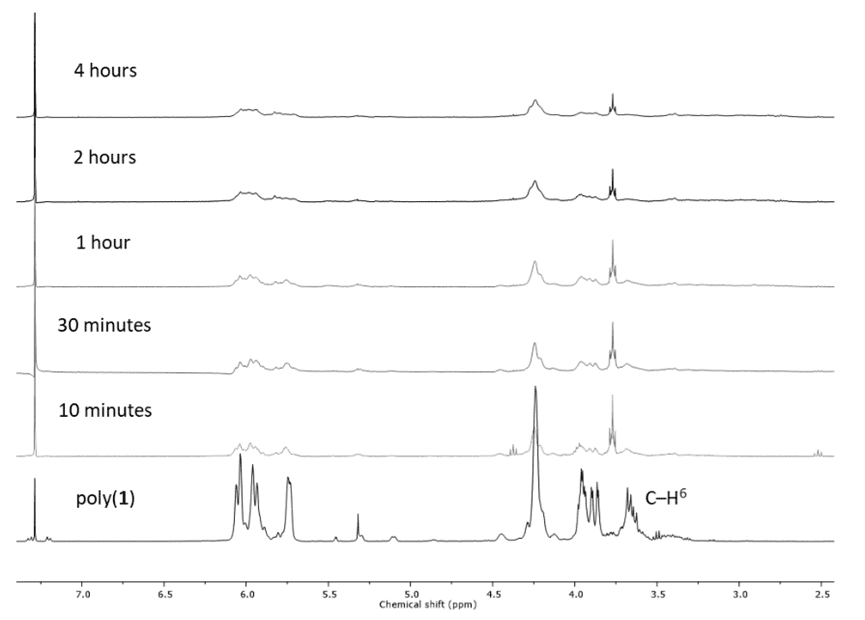
The polyxanthate structure can be degraded under UV illumination. NMR analysis of the UV-treated samples revealed that the thiocarbonate/thioester bond was broken, as shown in the figure below.
5. Summary
The authors synthesized a novel bio-based derived unsaturated cyclic xanthan monomer and successfully co-polymerized it with L-propyl cross-ester via ROP to form a series of poly(lactic acid) copolymers, which can degrade under UV irradiation. This new monomer can achieve improved environmental degradability of PLA without significantly affecting PLA performance.

