What is the lactide that makes PLA? Why is it always "stuck"?
1. Why is it so difficult to produce lactide?
Polylactic acid (PLA), also known as polylactide, has a molecular formula of (C3H4O2)n. It is a type of aliphatic polyester polymerized by the fermentation product of microorganisms-lactic acid as monomer. Corn, cassava, etc. as raw materials.
At present, the high energy consumption, high cost, insufficient yield and purity that still exist in the research on the preparation of polylactic acid intermediate-lactide are the main reasons for the excessively high cost of polylactic acid.
Some companies even shut down some production lines because they cannot purchase polylactic acid raw materials. Some manufacturers have insufficient supply of PLA due to the shortage of imported lactide. The price of lactide has remained high, and its production capacity has also become an important factor hindering the popularization of PLA.
In addition, the relative molecular mass of the polylactic acid product is still at a low level, which cannot meet the mechanical strength and strength retention time required by biological materials, which limits the application range of polylactic acid. Therefore, research on the polylactic acid process, optimization of the process flow, reduction of production costs, and improvement of the relative molecular weight of the product are of great significance for the promotion of the industrial production and application of polylactic acid.
So, what is lactide? How to prepare and produce? Is that really so difficult?
2.Lactic acid
To talk about the synthesis of lactide, we must first understand lactic acid.
Lactic acid is one of the simplest chiral molecules. Simply put, lactic acid has two optical isomers, D(+) and L(-). D and L-lactic acid, which is a mixture of equal amounts of D(+) and L(-), does not have optical rotation, so there are 3 types of lactic acid with different optical rotations.
The two isomer forms are shown in the figure:
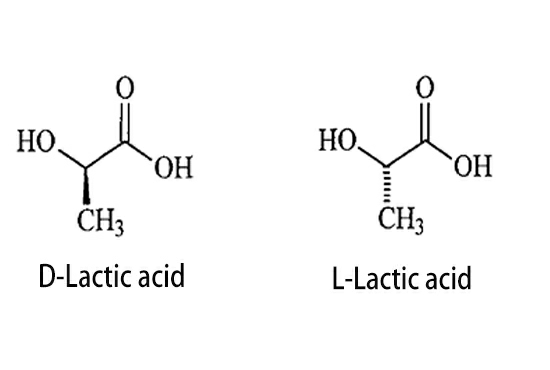
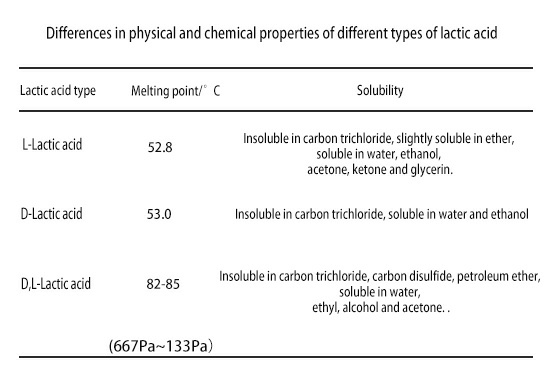
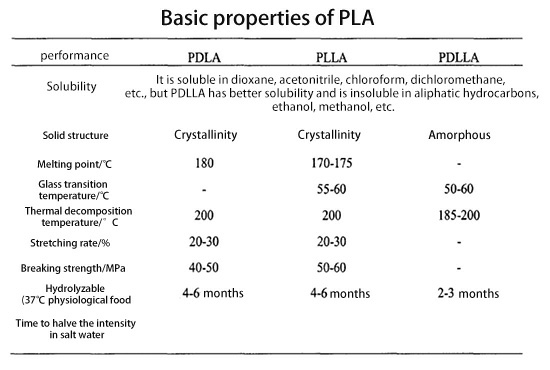
Due to the difference in conformation, different types of lactic acid have different physical and chemical properties, as shown in the table below:
3.Lactide
From the point of view of the raw materials for the synthesis of lactide, lactide is generally synthesized from lactide or lactic acid.
When using lactate as the raw material for the reaction, although the reaction time is faster and the product purity is high, the cost of lactate is too high, and the product is generally mixed with impurities such as acetic acid, which is difficult to purify, and the product yield is very low. There are very few laboratory research and industrial applications.
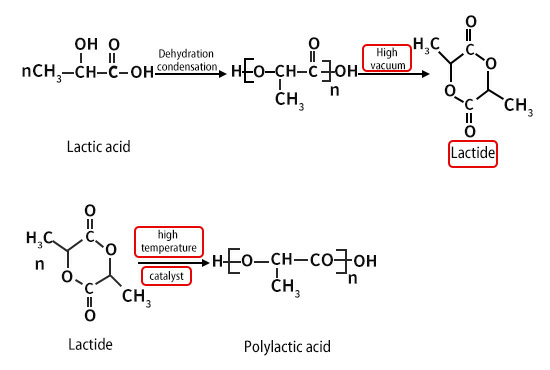
However, it is more economical to use lactic acid obtained by biological fermentation as a raw material for the reaction, and the product yield is higher, and the current research is more in-depth.The process flow is that lactide is firstly esterified and dehydrated to generate lactic acid oligomers, and then cracked at a higher temperature to catalyze depolymerization to obtain lactide.
The reaction formula is as follows:
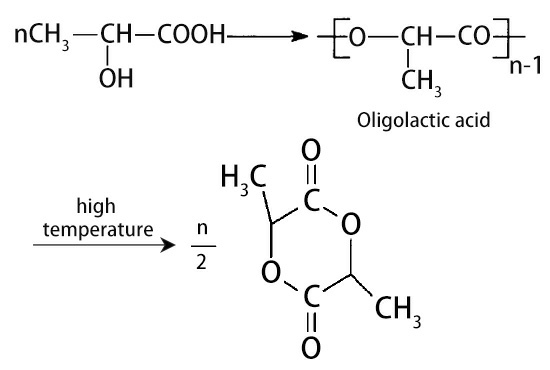
Lactide is a cyclic dimer of lactic acid. There are 4 types of lactide in terms of optical rotation:
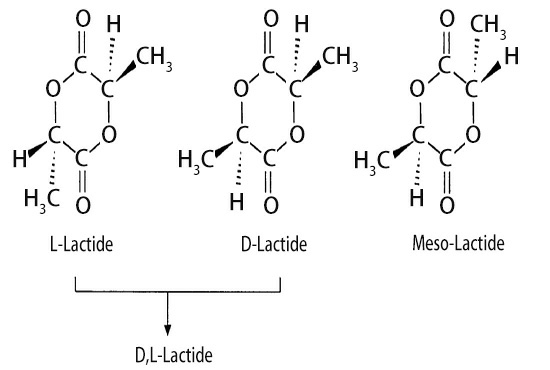
(1)The lactide formed by the dehydration of two L-lactic acid molecules is called L, L-lactide (or L-lactide for short);
(2)The lactide formed by the dehydration of two D-lactic acid molecules is called D, D-lactide (or D-lactide for short);
(3)The lactide formed by the dehydration of a L-lactic acid molecule and a D-lactic acid molecule is called meso D, L-lactide (also known as Meso-lactide);
(4)Equal amounts of L- and D-lactide (melting point 96°C) are mixed to form racemic D, L-lactide (melting point 127°C, hereinafter referred to as D, L-lactide).
Usually, the prepared lactide is a mixture of the above 4 kinds of lactide, but meso-lactide is easy to hydrolyze, hard to crystallize, and difficult to purify. In addition, the synthesized polylactic acid has low mechanical strength, and it is usually less used to synthesize polylactic acid.
As an important monomer in the synthesis process of polylactic acid, the purity and yield of lactide will directly affect the quality and production cost of polylactic acid. Because the crude lactide still contains impurities such as water, lactic acid, lactic acid oligomers, etc., it does not meet the requirements for the purity of the synthetic raw material of polylactic acid. Therefore, the crude lactide needs to be purified and refined.
Lactide must be purified to polymerize to obtain high molecular weight products, which is also the main reason for the high price of indirect methods.
4.The purification of lactide is difficult
There are two main methods for the purification of lactide:
(1)Recrystallization method
This method has complicated procedures and high solvent consumption. Solvent recrystallization is widely used in laboratories. The solvents used mainly include alcohols, ethers, esters, ketones, benzenes, halogenated alkanes and tetrahydrofuran. Because the yield of single-solvent single recrystallization is generally not high, a composite solvent multiple recrystallization method was developed to recrystallize lactide. The study found that recrystallization with ethyl acetate three times can effectively avoid the residue of hydroxyl in the product, but the recrystallization yield is low; however, the purity of lactide obtained by recrystallizing ethanol once cannot meet the requirements. Therefore, first recrystallize with ethanol twice to obtain a higher recrystallization yield, and then recrystallize with ethyl acetate once. It can effectively remove the residual hydroxyl in lactide, and make the yield of refined L-lactide reach 35.4%.Although the solvent recrystallization method is easy to operate, the volatile, flammable, explosive, and highly polluting characteristics of organic solvents limit the application of the solvent recrystallization method in industrial production.
(2)Vacuum distillation method
The equipment investment is large and the technical requirements are high.The advantage of the distillation method is that there is no need to use other types of solvents, so as to ensure that the purification and refining process will not introduce new impurities, and the equipment is easy to large-scale and continuous production. There are reports in the literature of a method of continuous rectification and purification of lactide, using a three-tower process: The vacuum operation of the first distillation tower removes low-boiling components at the top of the tower; Vacuum operation of the second distillation tower removes all meso-lactide at the top of the tower; The third distillation tower is operated in vacuum at the top of the tower to obtain high-purity lactide products, and the yield of lactide with a purity of more than 99% is higher than 90%.This process has strong industrial application potential.
5.The preparation of lactide is difficult
Commonly used methods for the preparation of lactide include atmospheric pressure method and reduced pressure method. The main problem faced by the two methods is how to reduce the coking and carbonization phenomenon that occurs in the depolymerization process of the reaction liquid, thereby increasing the yield of lactide. The design idea of the atmospheric pressure method is to pass "inert gas" into the reaction system, and with the help of these inert gases, the lactide produced is taken out of the reaction system. Japan’s Okuyama et al. used the atmospheric pressure method to prepare lactide, that is, to pass "inert gas flow" N2 or CO2 into the reaction system to reduce the partial pressure of lactide vapor and continuously remove the resulting lactide from the reaction system. Then Bring it out. The oxygen in the reactor is replaced by inert gas, which avoids side reactions such as discoloration and coking caused by oxidation, and improves the yield and purity of lactide to a certain extent. The method has low technical difficulty and high operation success, but the disadvantages of the atmospheric pressure method are long dehydration time, low production efficiency, and low product yield.
At present, the most commonly used method for the synthesis of lactide is the reduced pressure method. The design idea is to put the entire reaction system in a high vacuum state, and the resulting lactide can be quickly distilled out. In addition, under high vacuum, oxygen is isolated and reduced The occurrence of oxidation reaction increases the yield of lactide. On the basis of the reduced pressure method, the researchers found that adding a certain amount of "inert solvent" to the reaction system can well improve the phenomenon of easy coking and carbonization of the reaction liquid. For example, Hotsota and Li Nan added glycerin and higher alcohol solvents to the decompression system. They believe that these inert solvents can not only bring out the formed lactide, but also increase the viscosity of the reaction liquid in the late stage of the reaction. During the reaction process, side reactions will not occur due to excessive local temperature.
In addition to the above technological improvements, the researchers also studied the types of initiators and the relative molecular mass of oligomeric lactic acid. In addition to the above-mentioned tin-based initiators in the selection of catalysts, researchers have also used lactate and rare earth metal compounds for the reaction.
6.Influencing factors of lactide synthesis technology
(1)Temperature
The depolymerization of lactic acid oligomers is a reversible reaction. If the temperature of the depolymerization process is too low, the lactic acid oligomers are not easy to depolymerize to form lactide; if the temperature is too high, the lactic acid oligomers will decompose and oxidize. In addition, the formed lactide will also be discolored due to coking and carbonization and will be racemized. Generally, the depolymerization temperature is controlled at 220-250°C.
(2)Pressure
In order to promote the depolymerization to proceed in the direction of the positive reaction while avoiding the decomposition and discoloration of lactide, the lactide produced should be separated from the reaction system in time during the reaction. At present, there are more decompression methods and atmospheric pressure methods.
The pressure of the reaction system of the reduced pressure method should be lower than the vapor pressure of lactide at the reaction temperature. The decompression method reduces the oxygen content in the reaction system and can effectively avoid the discoloration of lactide caused by oxidation. However, the decompression method usually requires high vacuum equipment and the degree of vacuum is not easy to control, and the energy consumption in the process is relatively large.
At the end of the 1980s, people developed a process for preparing lactide at atmospheric pressure. The process maintained the pressure of the reaction system by introducing protective gas such as CO2 or N2 that did not participate in the reaction. The introduction of shielding gas helps to homogenize the reaction system, enlarges the reaction interface and improves the reaction efficiency. In addition, it can also reduce the oxygen content in the reaction system and quickly bring the produced lactide out of the reaction system. Therefore, the phenomenon of coking and carbonization of lactide is avoided.
However, the gas flowing out in the atmospheric pressure method will also take away part of the raw materials, resulting in a decrease in the yield. It is reported in the literature that using L-lactic acid as a raw material and passing N2 into the reaction system as a protective gas under normal pressure, the yield of crude lactide can reach 89%, which is significantly higher than the reduced pressure method.
(3)Catalyst
The depolymerization of lactic acid oligomers is an intramolecular transesterification reaction. The empty orbitals of metal elements can form coordination bonds with the carbonyl oxygen atoms of the lactic acid oligomers, thereby catalyzing the depolymerization reaction. At present, people have developed a variety of lactide synthesis catalysts, the main types are: metal compound catalysts, such as zinc, tin, aluminum, titanium, etc.; rare earth catalysts, such as yttrium, lanthanum, etc.; proton acid type catalysts, such as sulfuric acid, p-toluenesulfonic acid, solid super acid, etc. or a compound catalyst composed of the above-mentioned catalysts.
7.The four isomers of lactide correspond to synthetic polylactic acid including PLLA, PDLA, PDLLA and meso-PLA.
8.Synthetic method of polylactic acid
(1)Direct Polycondensation
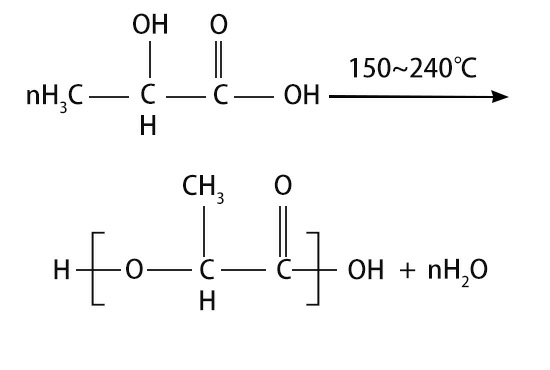
The direct polymerization of polylactic acid is a typical polycondensation reaction. There is an equilibrium reaction of free lactic acid, water, polyester and lactide in the reaction system. The polymerization equation is as follows:
The advantages of the direct polymerization method are:
(a)High yield, can get polylactic acid close to theoretical yield.
(b)The process starting from the finished lactic acid is short, the process is simple, and the synthesized polylactic acid does not contain a catalyst. Cost is lower than indirect polycondensation method.
The disadvantages are:
(a)The non-volatile impurities in the raw material lactic acid will eventually remain in the finished polylactic acid, so only pure lactic acid can be used as the raw material. Sugars and proteins involved in the lactic acid fermentation process must be removed.
Pure lactic acid must at least be water-white in appearance, otherwise the color will become darker during long-term heating and polymerization. If it is polylactic acid used as a medical material or food packaging, it is also required that the harmful metal ions in the raw material lactic acid do not exceed the standard. Therefore, the process of purifying lactic acid may be relatively long
(b)The molecular weight of the obtained polylactic acid is still in the lower limit of the usable range, and high molecular weight polylactic acid (such as degradable surgical sutures) cannot be prepared. The mechanical strength and strength retention time required by biological materials cannot be met. Limits its application areas.
The main reason for the low molecular weight of polylactic acid synthesized by the direct method is that the dehydration polycondensation process is a reversible reaction. The removal of water is the key factor of the whole process, after the reaction has progressed to a certain extent, a small amount of water in the system may cause the termination of the polycondensation reaction, thereby affecting the relative molecular mass of the final product. There are few studies on the preparation of polylactic acid by direct polycondensation, and there are still technical barriers, the reaction conditions are very harsh, and the process requirements are high.
(2)Indirect Polycondensation Method (lactide ring-opening polymerization method)
The preparation of high molecular weight polylactic acid is mainly through indirect polymerization. Lactic acid is first dehydrated and polymerized under certain conditions into low molecular weight lactic acid oligomers, and then cracked at a higher temperature to form an intermediate—lactide. The lactide is purified by recrystallization and then undergoes ring-opening polymerization under the action of an initiator. Becomes polylactic acid.
The reaction process is as follows:
For a long time, people have paid more attention to the ring-opening polymerization of lactide. This method is relatively easy to implement and can produce polylactic acid with a relative molecular mass of up to 700 to 1 million.
However, when the indirect method is adopted, the obtained crude lactide needs to be purified and refined, because the crude lactide still contains impurities such as water, lactic acid, and lactic acid oligomers, which does not meet the purity requirements of polylactic acid synthesis raw materials.Lactide must be purified to polymerize to obtain high molecular weight products, which is also the main reason for the high price of indirect methods.
Advantages of indirect polymerization method:
(a)High molecular weight polylactic acid can be obtained.
(b)Low-purity lactic acid can be used as raw material, and even scraps and waste can be used. This is because volatile lactide can be separated from non-volatile impurities (proteins, polysaccharides).Impurities can be further removed when the lactide is purified by recrystallization or distillation.
Disadvantages of indirect polymerization method:
(a)Lactide must be purified to polymerize to obtain high molecular weight products.
9.Summary
From the above analysis, the purity and yield of lactide will directly affect the quality and production cost of polylactic acid. In China, many manufacturers have mastered the preparation technology of lactide, but the purity and mass production are still need to further overcome difficulties.

